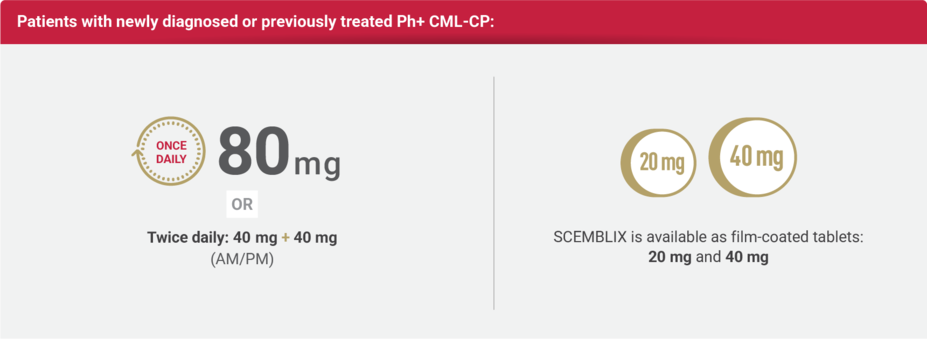
ORAL DOSING
Once-daily dosing option to accommodate your patients1
Patients should:
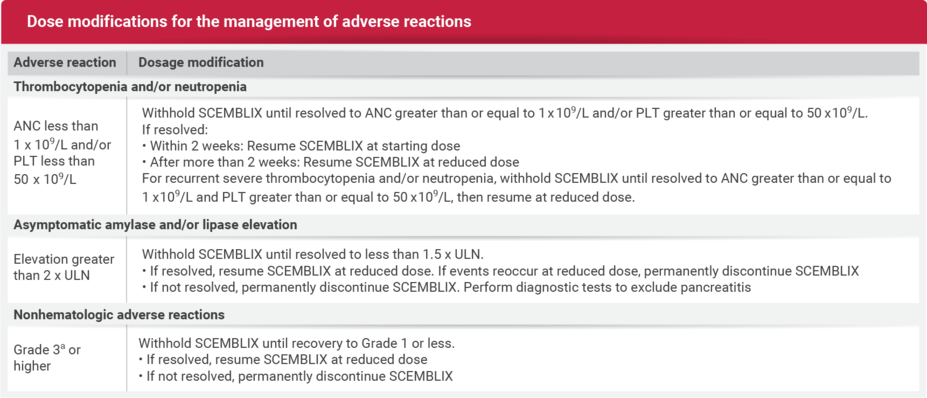
DOSAGE MODIFICATIONS
Dosage reductions and modifications
DOSAGE REDUCTIONS
For the management of ARs, reduce the SCEMBLIX dose as described in the table below.
• First reduction: 40 mg qd OR 20 mg bid
• Subsequent reduction: Permanently discontinue SCEMBLIX in patients unable to tolerate 40 mg qd OR 20 mg bid
DOSAGE MODIFICATIONS FOR THE MANAGEMENT OF ADVERSE REACTIONS
ANC, absolute neutrophil count; AR, adverse reaction; bid, twice daily; CTCAE, Common Terminology Criteria for Adverse Events; Ph+ CML-CP, Philadelphia chromosome–positive chronic myeloid leukemia in chronic phase; PLT, platelets; qd, once daily; ULN, upper limit of normal.
aBased on Common Terminology Criteria for Adverse Events (CTCAE) v4.03.