Review the ASC4FIRST study design
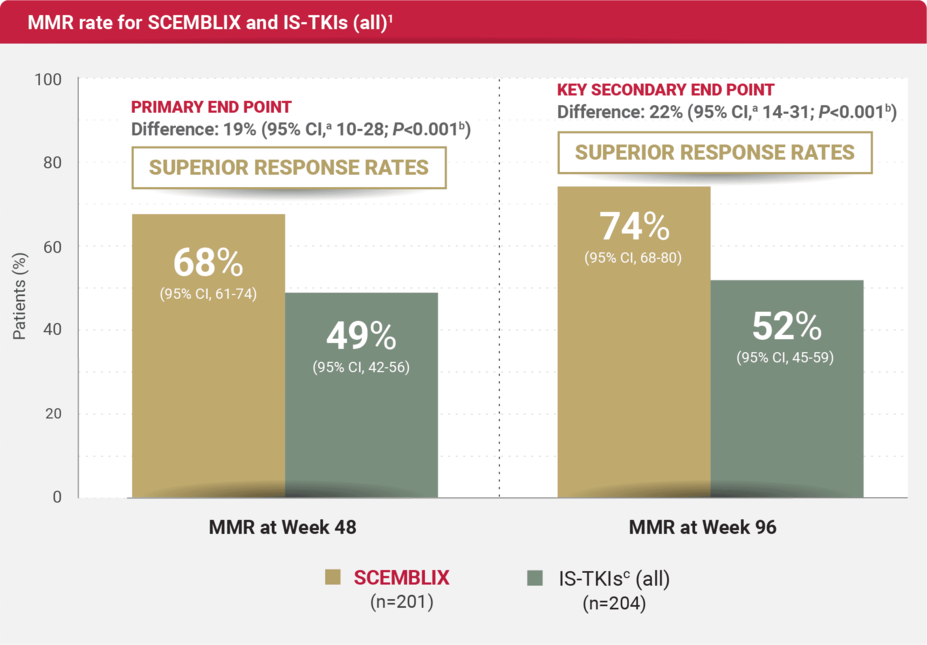
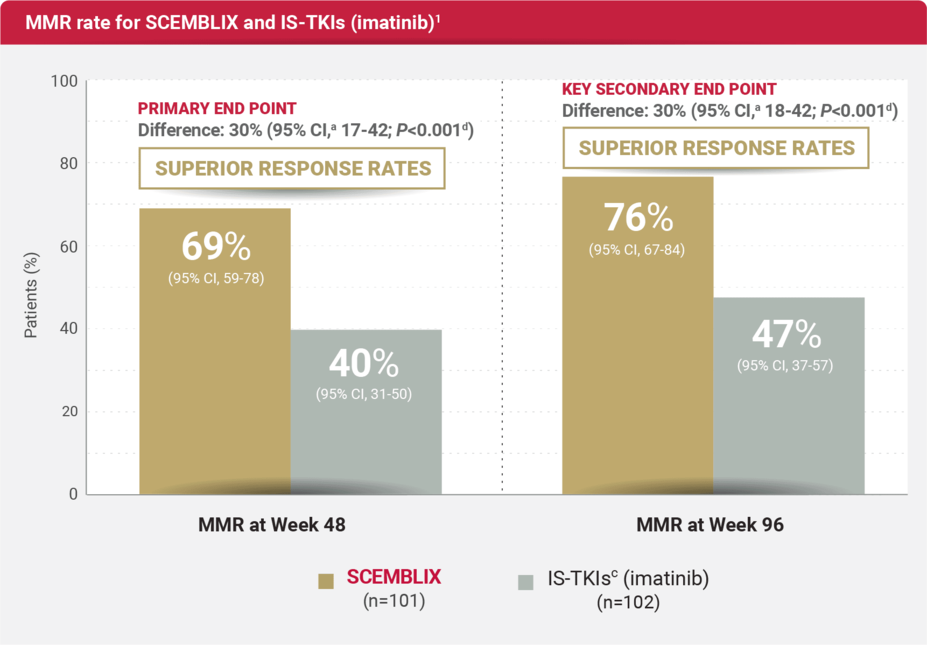
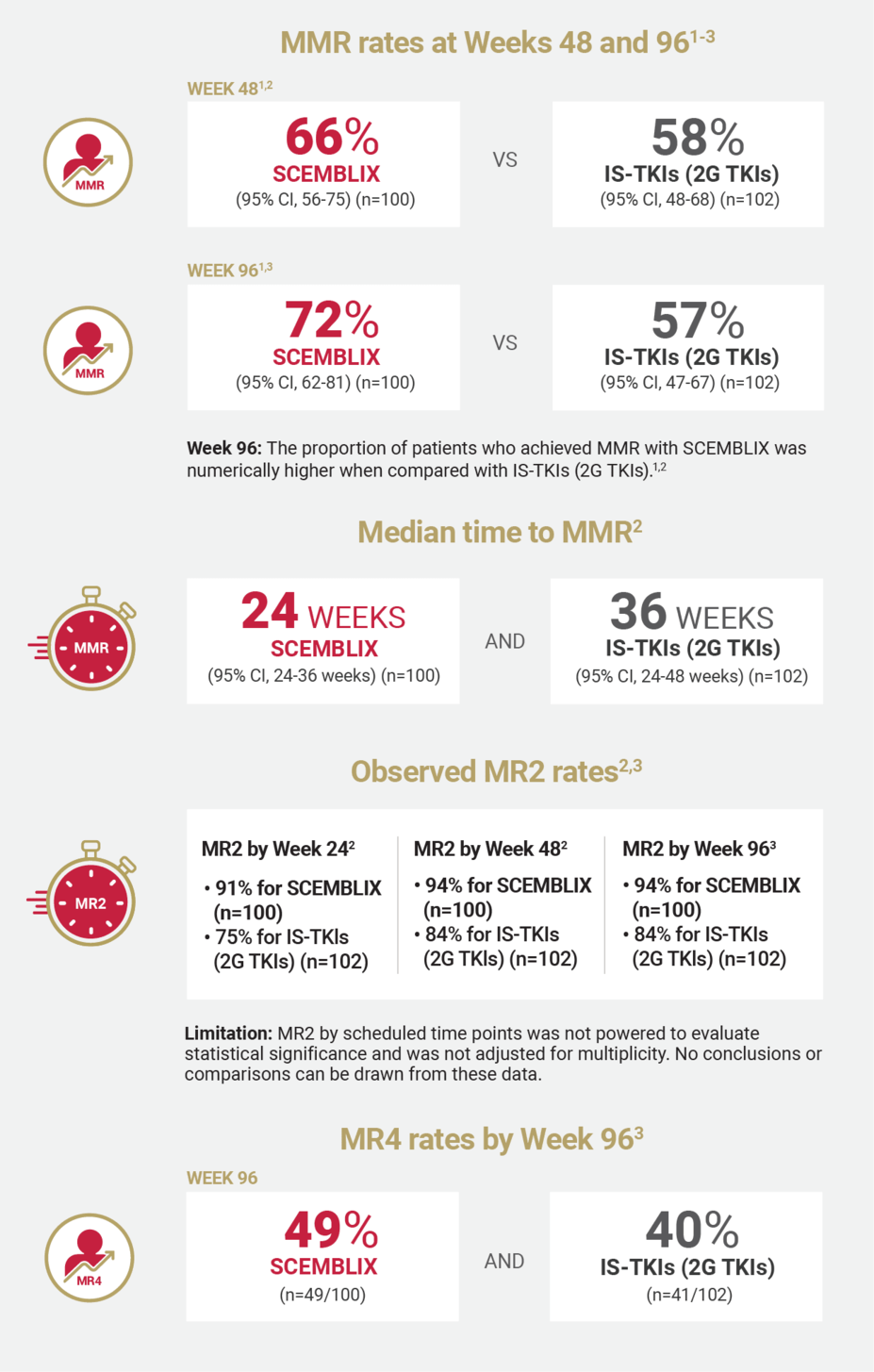
MMR RATES
For adults with newly diagnosed Ph+ CML-CP
SCEMBLIX delivered superior response rates at Week 48 and 961
aEstimated using a common risk difference stratified by PRS-TKI and baseline ELTS risk groups.1
bAdjusted P-value using a Cochran-Mantel-Haenszel 1-sided test stratified by PRS-TKI and baseline ELTS risk groups.1
cStandard-of-care (SoC) TKIs include imatinib (400 mg once daily) and other 2nd-generation TKIs: nilotinib (300 mg twice daily), dasatinib (100 mg once daily), or bosutinib (400 mg once daily).1
dAdjusted P-value using a Cochran-Mantel-Haenszel 1-sided test stratified by baseline ELTS risk groups.1
MMR was defined as BCR::ABL1IS ≤0.1% (≥3.0 log reduction).1,2
Median duration of follow-up3
At Week 96:
26.9 months for SCEMBLIX
26.3 months for IS-TKIs
Median duration of treatment1
At Week 96:
27 months (range, 0.2-36 months) for SCEMBLIX
25 months (range, 0.3-35 months) for IS-TKIs
MEDIAN TIME TO MMR
SCEMBLIX worked fast1
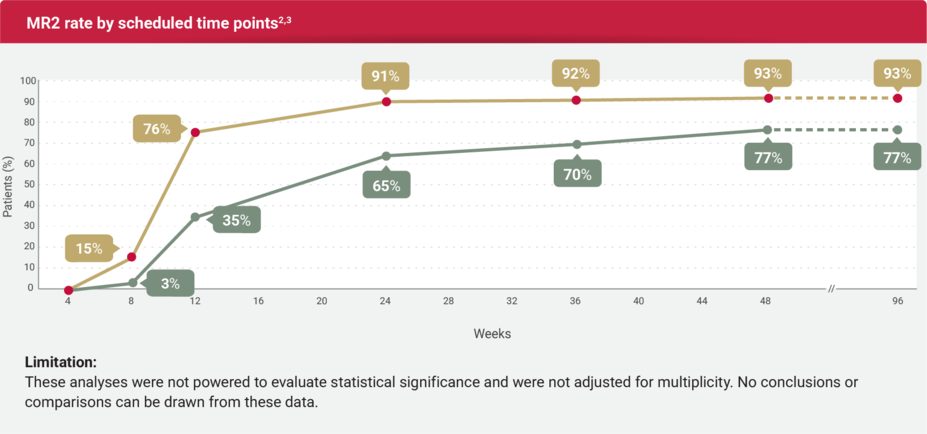
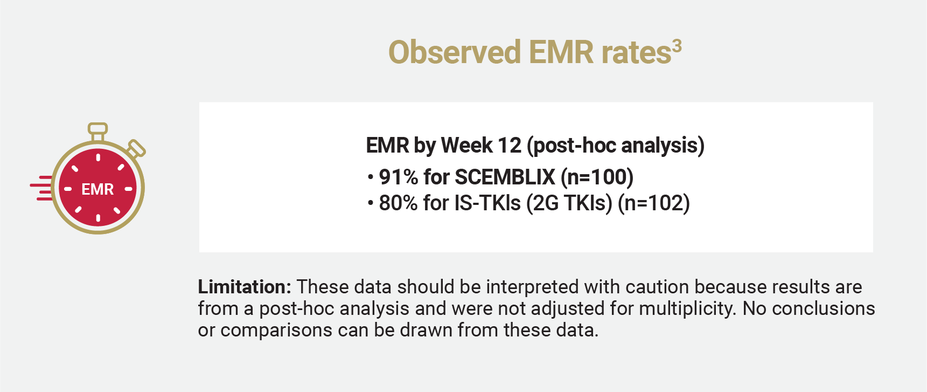
MR2 AND EMR RATES
Observed MR2 and EMR rates for SCEMBLIX and standard-of-care TKIs2,3
MR2 was defined as BCR::ABL1IS ≤1%.2
EMR was defined as BCR::ABL1IS ≤10% at 12 weeks.2
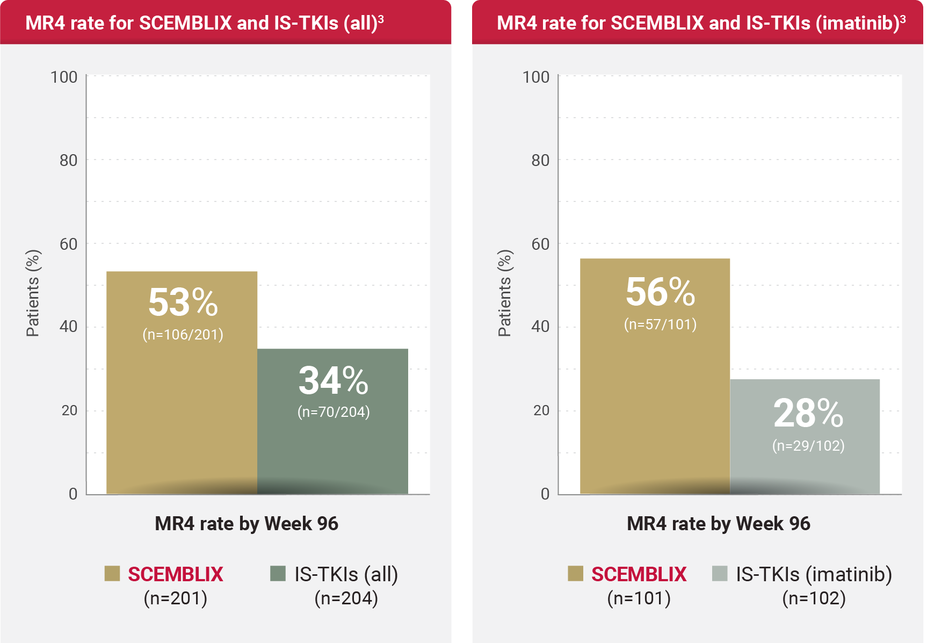
MR4 RATES
MR4 rates for SCEMBLIX and standard-of-care TKIs by Week 963
MR4 was defined as BCR::ABL1IS ≤0.01%.2
SCEMBLIX VS 2G TKIs
Efficacy for SCEMBLIX and standard-of-care TKIs (2G TKIs)1-3
Select other secondary end points and post-hoc analysis